Description
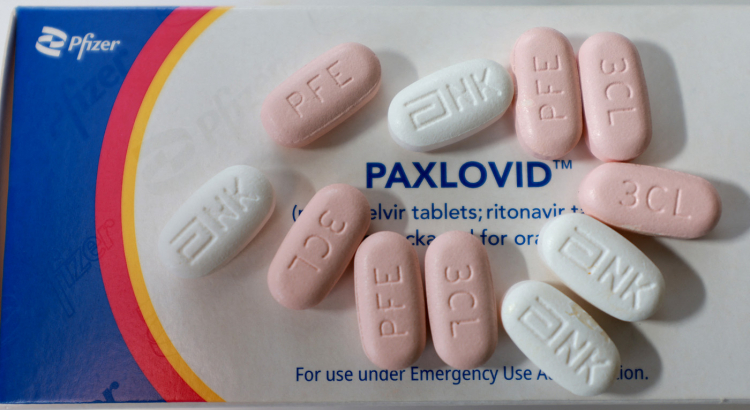
PAXLOVID For the treatment of mild-to-moderate COVID-19 in adults and pediatric patients (12 years of age and older weighing at least 40 kg) with positive results from direct SARS-CoV-2 viral testing and who are at high risk for progression to severe COVID-19, including hospitalization or death, PAXLOVID has not been approved but has been authorized for emergency use by the FDA under an EUA.
Only while it is determined that there are circumstances supporting the authorization of the emergency use of pharmaceuticals and biological products during the COVID-19 pandemic is PAXLOVID permitted for emergency use.


Reviews
There are no reviews yet.